Product News
LGM Pharma Invests $6M in U.S. Drug Manufacturing Capabilities
LGM Pharma announces a $6M investment to expand its Rosenberg, Texas facility, enhancing its capacity for liquid, semi-solid and suppository drug production. The expansion supports growing prescription drug demand.

Product News
BIOVECTRA Is Honored With 2025 CDMO Leadership Award for Biologics
BIOVECTRA, part of Agilent Technologies, has been awarded the 2025 Outsourced Pharma CDMO Leadership Award in biologics for its exceptional quality, reliability and technical capability.

Product News
Thermo Fisher Scientific Introduces TSX™ Core Series ULT Freezers
Thermo Fisher Scientific introduces the Thermo Scientific™ TSX™ Core Series ULT freezers, designed to provide reliable performance and robust durability in a user-friendly solution.

Product News
ProBioGen Successfully Completes CMC Development Milestones for Marea’s Promising Acromegaly Therapeutic
ProBioGen and Marea Therapeutics, Inc., a clinical-stage biotechnology company, announced the successful completion of a sprint project to deliver clinical material.
Product News
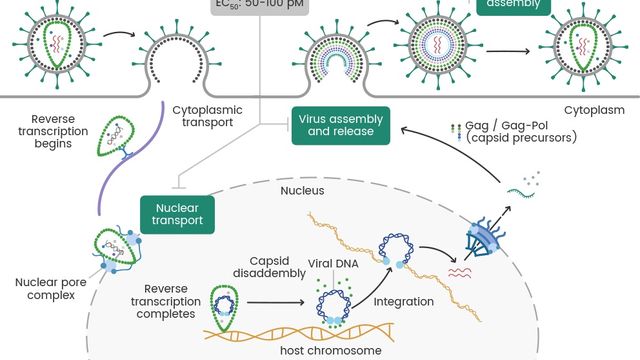
Gilead’s Capsid Revolution Meets Our Capsid Solutions: Sino Biological – Engineering the Tools To Outsmart HIV
On February 18, 2025, Gilead Sciences announced a significant milestone: the U.S. FDA accepted their New Drug Applications (NDAs) for lenacapavir (LEN), a twice-yearly injectable HIV-1 capsid inhibitor for pre-exposure prophylaxis (PrEP).
Product News
Celonic Group Signs Long-Term Multi-Year Manufacturing Service Agreement With LINDIS Biotech for the Commercial Supply of Catumaxomab
Celonic Group, a “Pure Play” biologics contract development and manufacturing organization (CDMO), announced the signing of a multi-year commercial manufacturing agreement with LINDIS Biotech.
Advertisement